particle in a box approximation metals • The particle in a box problem can be solved exactly and is a good first approximation for the electrons in a delocalized π-system. • Confining a particle in a box leads to quantization of its . In fact, the most appropriate wire size for 100 amp service is the #1 AWG wire with a 130 amp median capacity. To understand why this is, and what wire size you need for 100 amp sub panels 100 or 150 feet away, let’s look at the what the NEC code requirements are for 100 amp wire size:
0 · particle in a box wikipedia
1 · particle in a box model
2 · particle in a box function
3 · particle in a box examples
4 · particle in a box equation
5 · particle in a box
6 · particle in 2 dimensional box
7 · formula for particle in a box
$779.95
The particle in the box model system is the simplest non-trivial application of the Schrödinger equation, but one which illustrates many of the fundamental concepts of quantum mechanics. A particle in a 2-dimensional box is a fundamental quantum mechanical approximation describing the translational motion of a single particle confined inside an infinitely deep well from which it .
Because of its mathematical simplicity, the particle in a box model is used to find approximate solutions for more complex physical systems in which a particle is trapped in a narrow region of low electric potential between two high potential barriers. These quantum well systems are particularly important in optoelectronics, and are used in devices such as the quantum well laser, the quantum well infrared photodetectorThe particle in the box is a hypothetical situation with a particle trapped in a one-dimensional “box”. Let’s not get hung-up on the fact that the common object called a “box” is typically an object with three dimensions instead of just one .• The particle in a box problem can be solved exactly and is a good first approximation for the electrons in a delocalized π-system. • Confining a particle in a box leads to quantization of its .Explain why the energy of a quantum particle in a box is quantized; Describe the physical meaning of stationary solutions to Schrӧdinger’s equation and the connection of these solutions with time-dependent quantum states; Explain .
The Particle in a Box (PIB) is a simple model that helps illustrate the behavior of electrons confined within atoms and molecules. It serves as a useful tool to introduce key quantum concepts: Energetic Quantization : Energy levels in .Free particle and the particle in a box. Schrödinger equation is a 2nd-order diff. eq. 2 ∂2ψ ( x ) − + V ( x )ψ ( x Eψ ( x. ) 2m ∂x2. We can find two independent solutions φ. ( x ) and φ.Approximately, therefore the particle in a box in a finite potential energy well can be considered as a first (crude) approximation model of an atom. At least for the purpose of demonstrating the .
We will show how this relationship can be derived from the results of the 1D particle in a box. Additionally, we will show how the particle in a box model can be applied to make . In these applications, the mathematical formalism of the particle-in-a-box model provides a foundation for understanding the quantum behavior of particles in confined systems. Real-world systems may require more .The simplest is a one-dimensional “particle in a box” problem. . This turns out to be quite a good approximation for electrons in a long molecule, and the three-dimensional version is a reasonable picture for electrons in metals. . It now becomes obvious that if the box does not have infinite walls, but merely high ones, . The Particle-In-A-Box approximation. Electrons in the \( \pi \)-electron system of a conjugated aromatic compound are not restricted to specific nuclei but are free to move throughout the system. In a linear conjugated .
Step 1: Define the Potential Energy V. The potential energy is 0 inside the box (V=0 for 0L). We assume the walls have infinite potential energy to ensure that the particle has zero probability of . The quantum particle in the 1D box problem can be expanded to consider a particle within a higher dimensions as demonstrated elsewhere for a quantum particle in a 2D box.Here we continue the expansion into a particle trapped in a 3D box with three lengths \(L_x\), \(L_y\), and \(L_z\). As with the other systems, there is NO FORCE (i.e., no potential) acting on .
The particle-in-a-well (= 1D box) is defined by having infinite potential energy outside the walls and a constant finite energy within. That turns out to be a very good approximation of the potential energy function of a pi system or a line of identical metal atoms. At the end of the line of atoms, an electron faces a steep potential energy curve.Particle in a Box and the Real World Chapter 16 The particle in a 1D box system: n = 1,2 ,3. BC: (0) = 0 and (a) = 0 The potential energy function of the 1D box is an infinitely deep well. An infinitely ‘deep’ potential well is only a theoretical construct and do not look like any real system. A reasonable approximation for this distance can be obtained by assuming that all of the carbon–carbon bonds are of equal length, leading to. L = (k + 1) (l C−C) + l C−N + l C═N (2) . The particle-in-a-box (PB) model works well for cyanine dyes and other conjugated linear systems with little or no bond-length alternation not because a .In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes the movement of a free particle in a small space surrounded by impenetrable barriers.The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example, a .
Answer to 17. The molecular orbital energies of butadiene. 17. The molecular orbital energies of butadiene CH2=CH-CH=CH2 can be ap- proximately represented either using the particle-in-a-box model or using the Hückel approximation (although the latter approximation significantly under- estimates the energies of anti-bonding molecular orbitals).We now turn our attention to a generalization of the 1-dimensional quantum box to 3 dimensions. The 3 - dimensional quantum box is shown schematically in figure 1.5. It extends along 0 ^ x ^ L x, 0 ^ у ^ L y, 0 ^ z ^ L z. Outside this region the potential is infinite so that the wavefunction ¥ is zero at the faces of the box.Answer to Based on the particle in a one-dimensional box. Science; Chemistry; Chemistry questions and answers; Based on the particle in a one-dimensional box approximation for polyenes, suggest where along the line segment the n=1 to n=2 electronic transiton would most likely take place. explain your choice
china cnc machining tolerance 0.01 suppliers
9 Particle-in-a-box(PIB) 1. Consider a linear poly-ene. 2. Theelectrons are completelydelocalized insidethepoly-ene, butcannotleavethe molecular framework. 3. Let us approximate this system by a one-dimensional box, of length L. The potential energy of the electrons inside the polyenes can be approximated by the figure below. 4.
particle in a box wikipedia
Question: To a crude first approximation a pi electron in a linear polyene may be considered as a particle in a 1D box. To a crude first approximation a pi electron in a linear polyene may be considered as a particle in a 1 D box. Here’s the best way to solve it. Solution.
Notice that the dimensionless Schrödinger equation contains no memory of the particle mass or the box length - this information is contained in the coordinate transformation \(x=yL/2\) and the natural energy. The goal will be to solve the dimensionless form of the problem and then restore units for the particular mass and box at the end. So each level of the particle in a box can contain two electrons, one spin up and the other spin down. Figure 3 shows how pi electrons for dye A (a 6 electron system) and dye B (a 8 electron system) are contained in the box . It states that we cannot know both the position and momemtum of a quantum particle with complete certainty. We will show how this relationship can be derived from the results of the 1D particle in a box. Additionally, we will show how the particle in a box model can be applied to make predictions on real systems. 4.5.5.2. Learning Goals:#
china cnc machining turning parts supplier
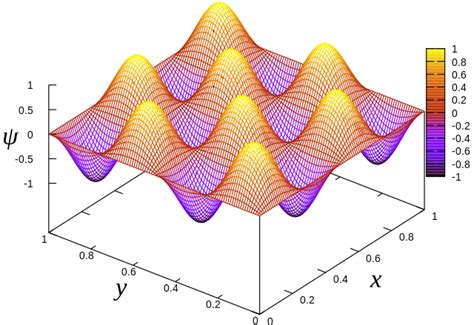
Luckily I found a source containing your molecule as an example.. You need your equation for the energy difference between the HOMO and the LUMO, which is (nearly) defined as you wrote: $$\Delta E = \frac{(N+1)h^2}{8 m_e L^2}$$ As you want a wavelength, you need to convert the energy difference, which yields then: $$\lambda = \frac{8m_ecL^2}{h(N+1)}$$ .Figure \(\PageIndex{2}\): Visualizing the first six wavefunctions and associated probability densities for a particle in a two-dimensional square box (\(L_x=L_y=L\)).Use the slide bar to independently change either \(n_x\) or \(n_y\) quantum number and see the changing wavefunction. Unlike in the one-dimensional analoge, where nodes in the wavefunction are .Consider a particle placed inside a 1D potential box Inside the box the potential energy V(x) is 0 . Metal InGaAs QW Band diagram in equilibrium The HEMT operates like a MOS transistor: The application of a positive or negative bias on the gate can increase or decrease the electronThe conventional quantum mechanical problem of a particle in a one-dimensional box can be made more interesting chemically without introducing any more difficult mathematics by employing a potential diagram rather than the usual infinite well.
This turns out to be quite a good approximation for electrons in a long molecule, and the three-dimensional version is a reasonable picture for electrons in metals. . However, it is not difficult to show that the total probability of finding the particle somewhere in the box remains unity. The Time-Independent Schrödinger Equation .Although a particle in such potential is an idealization, it is a very important problem because: a) Exact solutions are obtained from the Schrodinger Equation. b) It demonstrates important features of quantum-mechanical problems. c) This potential is a good approximation to some real situations such as a free electron in a metal.The particle-in-a-box type problems provide important models for several relevant chemical situations. . This same spherical box model has also been used to describe the valence electrons in quasi-spherical nano-clusters of metal atoms such as \(Cs_n\), \(Cu_n\), \(Na_n\), \(Au_n\), \(Ag_n\), and their positive and negative ions. Because of .Particle in a box with finite-potential walls. Contents . 2.6.1. Definition of the finte square well potential . We saw when looking at the Photoelectric effect that a reasonable approximation for the potential that confines the electrons within the metal had a finite depth. This is the potential we will now consider and it is be far the most .
Although a particle in such potential is an idealization, it is a very important problem because: a) Exact solutions are obtained from the Schrodinger Equation. b) It demonstrates important features of quantum-mechanical problems. c) This potential is a good approximation to some real situations such as a free electron in a metal.For the particle in a 1D box, we see that the number of nodes is equal to n−1. Thoughtheparticle in a1D boxisasimple model system, it illustratesthe important features of a quantum mechanical description. It is a very useful first approximation to the .
particle in a box model
We offer an extensive selection of plastic, concrete, polymer concrete, and composite underground pull boxes and handholes in variety of sizes. Our underground pull boxes are strong and durable, with the broadest and most complete line offered in the industry.
particle in a box approximation metals|particle in a box wikipedia